Cratylus What is in a name namely in the name name namely named

Terminology[edit source]
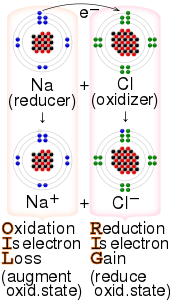
"Redox" is a combination of the words "reduction" and "oxidation". The term "redox" was first used in 1928.[5] The processes of oxidation and reduction occur simultaneously and cannot occur independently.[4] In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized, and the oxidant or oxidizing agent gains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a redox pair. A redox couple is a reducing species and its corresponding oxidizing form,[6] e.g., Fe2+
/ Fe3+
.The oxidation alone and the reduction alone are each called a half-reaction because two half-reactions always occur together to form a whole reaction.
Substances that have the ability to reduce other substances (cause them to gain electrons) are said to be reductive or reducing and are known as reducing agents, reductants, or reducers.
An electrolyte is a medium containing ions that is electrically conducting through the movement of ions, but not conducting electrons.[1][2][3] This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.[4][5]
(disambiguation).
ElectrochemistryEdit...In chemistry, ion association is a chemical reaction whereby ions of opposite electrical charge come together in solution to form a distinct chemical entity.[1][2] Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy.[3][4]
Classification of ion pairs..
Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be positive or negative (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects..The coulombic energy released when ions associate contributes to the enthalpy term, {}}
. In the case of contact ion pairs, the covalent interaction energy also contributes to the enthalpy, as does the energy of displacing a solvent molecule from the solvation shell of the cation or anion. The tendency to associate is opposed by the entropy term, which results from the fact that the solution containing unassociated ions is more disordered than a solution containing associates.
LanguageDownload PDFWatchEdit
The elementary charge, usually denoted by e or sometimes qe is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 e.[2] This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called the elementary positive charge The entropy term is similar for electrolytes of the same type, with minor differences due to solvation effects. Therefore, it is the magnitude of the enthalpy term that mostly determines the extent of ion association for a given electrolyte type. This explains the general rules given above.Occurrence
In order to achieve high ionic conductivity, electrochemical measurements are conducted in the presence of excess electrolyte. In water the electrolyte is often a simple salt such as potassium chloride. For measurements in nonaqueous solutions, salts composed of both lipophilic cations and anions are employed, e.g., tetrabutylammonium hexafluorophosphate. Even in such cases potentials are influenced by ion-pairing, an effect that is accentuated in solvents of low dielectric constant.[3]
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge.[1] A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes.[clarification needed] Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) or synthetic polymers (e.g., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups. A substance that dissociates into ions in solution or in the melt acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate in a liquid phase are examples of electrolytes.
electron, which has charge −1 e.[2] This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called the elementary positive charge.
Elementary electric chargeDefinition:Charge of a protonSymbol:e or sometimes qeValue in coulombs:1.602176634×10−19 C[1]
In the SI system of units, the value of the elementary charge is exactly defined {\displaystyle e\equiv } 1.602176634×10−19 coulombs.[1] Since the 2019 redefinition of SI base units, which took effect on 20 May 2019, the SI contains seven base units which are defined by seven fundamental physical constants. The elementary charge is one of these constants, and it enters in the definition of the base unit ampere (coulombs per second) through A ≡ e / (1.602176634×10−19 s).
The Eeee The electron is a subatomic particle (denoted by the symbol
e−
or
β−
or {{ce {^{0}{-1}e}}} whose electric charge is negative one elementary charge.[9] Electrons belong to the first generation of the lepton particle family,[10] and are generally thought to be elementary particles because they have no known components or substructure.[1] The electron has a mass that is approximately 1/1836 that of the proton.[11] Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. elementary charge.[9] Electrons belong to the first generation of the lepton particle family,[10] and are generally thought to be elementary particles because they have no known components or substructure.[1] The electron has a mass that is approximately 1/1836 that of the proton.[11] Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[10] Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.Even among particle physicists, the exact definition of a particle has diverse descriptions. These professional attempts at the definition of a particle include:
A particle is a collapsed wave functionA particle is a quantum excitation of a fieldA particle is an irreducible representation of the Poincaré groupA particle might be a vibrating stringA particle is a thing measured in a detector[6]
The reductant (reducing agent) transfers electrons to another substance and is thus itself oxidized. And, because it donates electrons, the reducing agent is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors. The word reduction originally referred to the loss in weight upon heating a metallic ore such as a metal oxide to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier demonstrated that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to include all processes involving a gain of electrons. Reducing equivalent refers to chemical species which transfer the equivalent of one electron in redox reactions. The term is common on biochemistry.[7] A reducing equivalent can be an electron, a hydrogen atom, as a hydride ion.[8]
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents. These metals donate or give away electrons relatively readily. They transfer electrons.
Hydride transfer reagents, such as NaBH4 and LiAlH4, reduce by atom transfer: they transfer the equivalent of hydride or H-. These reagents widely used in [the reduction of carbonyl compounds to alcohols.[9][10] A related method of reduction involves the use of hydrogen gas (H2) as sources of H atoms.
Oxidants[edit source]
Oxidation originally implied reaction with oxygen to form an oxide. Later, the term was expanded to encompass oxygen-like substances that accomplished parallel chemical reactions. Ultimately, the meaning was generalized to include all processes involving the loss of electrons. Substances that have the ability to oxidize other substances (cause them to lose electrons) are said to be oxidative or oxidizing, and are known as oxidizing agents, oxidants, or oxidizers. The oxidant (oxidizing agent) removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, the oxidizing agent is also called an electron acceptor. Oxidants are usually chemical substances with elements in high oxidation states (e.g., H
2O
2, MnO−
4, CrO
3, Cr
2O2−
7, OsO
4), or else highly electronegative elements (O2, F2, Cl2, Br2) that can gain extra electrons by oxidizing another substance.[citation needed]
Oxidizers are oxidants but the term is mainly reserved for sources of oxygen, particularly in the context of explosions. Nitric acid is an oxidizer.
Oxygen is the quintessential oxidizer.
Theosophy[edit source]
Besant was a prolific writer and a powerful orator.[21] In 1889, she was asked to write a review for the Pall Mall Gazette[22] on The Secret Doctrine, a book by H. P. Blavatsky. After reading it, she sought an interview with its author, meeting Blavatsky in Paris. In this way, she was converted to Theosophy. Besant's intellectual journey had always involved a spiritual dimension, a quest for transformation of the whole person. As her interest in theosophy deepened, she allowed her membership of the Fabian Society to lapse (1890) and broke her links with the Marxists. In her Autobiography, Besant follows her chapter on "Socialism" with "Through Storm to Peace", the peace of Theosophy. In 1888, she described herself as "marching toward the Theosophy" that would be the "glory" of her life. Besant had found the economic side of life lacking a spiritual dimension, so she searched for a belief based on "Love". She found this in Theosophy, so she joined the Theosophical Society, a move that distanced her from Bradlaugh and other former activist co-workers.[23] When Blavatsky died in 1891, Besant was left as one of the leading figures in theosophy and in 1893 she represented it at the Chicago World Fair.[24] Another activity in this period was her involvement in the London matchgirls strike of 1888. She was drawn into this battle of the "New Unionism" by a young socialist, Herbert Burrows. He had made contact with workers at Bryant and May's match factory in Bow, London, who were mainly young women and were very poorly paid. They were also prey to industrial illnesses, like the bone-rotting Phossy jaw, which was caused by the chemicals used in match manufacture.[17] Some of the match workers asked for help from Burrows and Besant in setting up a union.
Besant met the women and set up a committee, which led the women into a strike for better pay and conditions, an action that won public support. Besant led demonstrations by "match-girls", who were cheered in the streets, and prominent churchmen wrote in their support. In just over a week they forced the firm to improve pay and conditions. Besant then helped them to set up a proper union and a social centre.
At the time, the matchstick industry was a very powerful lobby, since electric light was not yet widely available, and matches were an essential commodity; in 1872, lobbyists from the match industry had persuaded the British government to change its planned tax policy. Besant's campaign was the first time anyone had successfully challenged the match manufacturers on a major issue and was seen as a landmark victory of the early years of British Socialism.
In 1884, Besant had developed a very close friendship with Edward Aveling, a young socialist teacher who lived in her house for a time. Aveling was a scholarly figure and it was he who first translated the important works of Marx into English. He eventually went to live with Eleanor Marx, daughter of Karl Marx. Aveling was a great influence on Besant's thinking and she supported his work, yet she moved towards the rival Fabians at that time. Aveling and Eleanor Marx had joined the Marxist Social Democratic Federation and then the Socialist League, a small Marxist splinter group which formed around the artist William Morris.
It seems that Morris played a large part in converting Besant to Marxism, but it was to the SDF, not his Socialist League, that she turned in 1888. She remained a member for a number of years and became one of its best speakers. She was still a member of the Fabian Society; neither she nor anyone else seemed to think the two movements incompatible at the time.
Soon after joining the Marxists, Besant was elected to the London School Board in 1888.[18] Women at that time were not able to take part in parliamentary politics but had been brought into the local electorate in 1881.
Annie Besant (née Wood; 1 October 1847 – 20 September 1933) was a British socialist, theosophist, women's rights activist, writer, orator, political party member, educationist, and philanthropist. Regarded as a champion of human freedom, she was an ardent supporter of both Irish and Indian self-rule. She was also a prolific author with over three hundred books and pamphlets to her credit. As an educationist, her contributions included being one of the founders of the Banaras Hindu University. For fifteen years, Besant was a public proponent in England of atheism and scientific materialism. Besant's goal was to provide employment, better living conditions, and proper education for the poor.[1] Annie Wood was born on 1 October 1847 in London into an upper-middle-class family. She was the daughter of William Burton Persse Wood (1816–1852) and Emily Roche Morris (died 1874). The Woods originated from Devon and her great-uncle was the Whig politician Sir Matthew Wood, 1st Baronet from whom derives the Page Wood baronets. Her father was an Englishman who lived in Dublin and attained a medical degree, having attended Trinity College Dublin. Her mother was an Irish Catholic, from a family of more modest means. Besant would go on to make much of her Irish ancestry and supported the cause of Irish self-rule throughout her adult life. Annie's father died when she was five years old, leaving the family almost penniless. Her mother supported the family by running a boarding house for boys at Harrow School. However, she was unable to support Annie and persuaded her friend Ellen Marryat to care for her. Marryat made sure that she had a good education. Annie was given a strong sense of duty to society and an equally strong sense of what independent women could achieve.[2] As a young woman, she was also able to travel widely in Europe. There she acquired a taste for Roman Catholic colour and ceremony that never left her.
In 1867, at age twenty, she married 26-year-old clergyman Frank Besant (1840–1917), younger brother of Walter Besant. He was an evangelical Anglican who seemed to share many of her concerns.[2] On the eve of her marriage, she had become more politicised through a visit to friends in Manchester, who brought her into contact with both English radicals and the Manchester Martyrs of the Irish Republican Fenian Brotherhood,[3] as well as with the conditions of the urban poor.
Marischal College, Aberdeen, 1856–1860
The 25-year-old Maxwell was a good 15 years younger than any other professor at Marischal. He engaged himself with his new responsibilities as head of a department, devising the syllabus and preparing lectures.[56] He committed himself to lecturing 15 hours a week, including a weekly pro bono lecture to the local working men's college.[56] He lived in Aberdeen with his cousin William Dyce Cay, a Scottish civil engineer, during the six months of the academic year and spent the summers at Glenlair, which he had inherited from his father.[14]
He focused his attention on a problem that had eluded scientists for 200 years: the nature of Saturn's rings. It was unknown how they could remain stable without breaking up, drifting away or crashing into Saturn.[57] The problem took on a particular resonance at that time because St John's College, Cambridge had chosen it as the topic for the 1857 Adams Prize.[58] Maxwell devoted two years to studying the problem, proving that a regular solid ring could not be stable, while a fluid ring would be forced by wave action to break up into blobs. Since neither was observed, he concluded that the rings must be composed of numerous small particles he called "brick-bats", each independently orbiting Saturn.[58] Maxwell was awarded the £130 Adams Prize in 1859 for his essay "On the stability of the motion of Saturn's rings";[59] he was the only entrant to have made enough headway to submit an entry.[60] His work was so detailed and convincing that when George Biddell Airy read it he commented "It is one of the most remarkable applications of mathematics to physics that I have ever seen."[1] It was considered the final word on the issue until direct observations by the Voyager flybys of the 1980s confirmed Maxwell's prediction that the rings were composed of particles.[61] It is now understood, however, that the rings' particles are not stable at all, being pulled by gravity onto Saturn. The rings are expected to vanish entirely over the next 300 million years.[62]
With the publication of "A Dynamical Theory of the Electromagnetic Field" in 1865, Maxwell demonstrated that electric and magnetic fields travel through space as waves moving at the speed of light. He proposed that light is an undulation in the same medium that is the cause of electric and magnetic phenomena.[4] The unification of light and electrical phenomena led his prediction of the existence of radio waves. Maxwell is also regarded as a founder of the modern field of electrical engineering.[5]
Electron acceptors[edit source]Physical properties[edit source]
Triphenylamine has three aromatic groups directly linked to the central nitrogen atom. Each aromatic group acts as an electron attractor, directing the electron cloud of the lone pair of nitrogen towards it. With the delocalization of the nitrogen lone pair,[4] a partial positive charge is conferred to nitrogen, counterbalanced by the partial negative charge localized on the aromatic groups. This arrangement prevents nitrogen protonation, a key mechanism for providing basicity to a solution.
 From this characteristic, moreover, it follows that the three N-C bonds all lie on the same plane and that they are located at 120° from each other, which is not the case with aliphatic amines and ammonia, where the orbitals of nitrogen are arranged in a tetrahedron. Due to steric hindrance, the phenyl groups are not on the same plane defined by the three N-C bonds, but are twisted, giving the molecule its characteristic "propeller-like" shape.
From this characteristic, moreover, it follows that the three N-C bonds all lie on the same plane and that they are located at 120° from each other, which is not the case with aliphatic amines and ammonia, where the orbitals of nitrogen are arranged in a tetrahedron. Due to steric hindrance, the phenyl groups are not on the same plane defined by the three N-C bonds, but are twisted, giving the molecule its characteristic "propeller-like" shape.
Electron acceptors participate in electron-transfer reactions. In this context, the oxidizing agent is called an electron acceptor and the reducing agent is called an electron donor. A classic oxidizing agent is the ferrocenium ion Fe(C
5H
5)+
2, which accepts an electron to form Fe(C5H5)2. One of the strongest acceptors commercially available is "Magic blue", the radical cation derived from N(C6H4-4-Br)3.[2]
Extensive tabulations of ranking the electron accepting properties of various reagents (redox potentials) are available, see Standard electrode potential (data page).
Classification[edit source]
Organic compounds may be classified in a variety of ways. One major distinction is between natural and synthetic compounds. Organic compounds can also be classified or subdivided by the presence of heteroatoms, e.g., organometallic compounds, which feature bonds between carbon and a metal, and organophosphorus compounds, which feature bonds between carbon and a phosphorus.
Another distinction, based on the size of organic compounds, distinguishes between small molecules and polymers.
Natural compounds[edit source]
Natural compounds refer to those that are produced by plants or animals. Many of these are still extracted from natural sources because they would be more expensive to produce artificially. Examples include most sugars, some alkaloids and terpenoids, certain nutrients such as vitamin B12, and, in general, those natural products with large or stereoisometrically complicated molecules present in reasonable concentrations in living organisms.
Further compounds of prime importance in biochemistry are antigens, carbohydrates, enzymes, hormones, lipids and fatty acids, neurotransmitters, nucleic acids, proteins, peptides and amino acids, lectins, vitamins, and fats and oils.
Hydraulics (from Greek: Υδραυλική) is a technology and applied science using engineering, chemistry, and other sciences involving the mechanical properties and use of liquids. At a very basic level, hydraulics is the liquid counterpart of pneumatics, which concerns gases. Fluid mechanics provides the theoretical foundation for hydraulics, which focuses on the applied engineering using the properties of fluids. In its fluid power applications, hydraulics is used for the generation, control, and transmission of power by the use of pressurized liquids. Hydraulic topics range through some parts of science and most of engineering modules, and cover concepts such as pipe flow, dam design, fluidics and fluid control circuitry. The principles of hydraulics are in use naturally in the human body within the vascular system and erectile tissue.[2][3] Free surface hydraulics is the branch of hydraulics dealing with free surface flow, such as occurring in rivers, canals, lakes, estuaries and seas. Its sub-field open-channel flow studies the flow in open channels.
The word "hydraulics" originates from the Greek word ὑδραυλικός (hydraulikos) which in turn originates from ὕδωρ (hydor, Greek for water) and αὐλός (aulos, meaning pipe).[4]
Second generation[edit source]
In the period between the two World Wars, the "Second Generation" Fabians, including the writers R. H. Tawney, G. D. H. Cole and Harold Laski, continued to be a major influence on socialist thought.
It was at this time that many of the future leaders of the Third World were exposed to Fabian thought, most notably India's Jawaharlal Nehru, who subsequently framed economic policy for India on Fabian socialism lines. After independence from Britain, Nehru's Fabian ideas committed India to an economy in which the state owned, operated and controlled means of production, in particular key heavy industrial sectors such as steel, telecommunications, transportation, electricity generation, mining and real estate development. Private activity, property rights and entrepreneurship were discouraged or regulated through permits, nationalisation of economic activity and high taxes were encouraged, rationing, control of individual choices and Mahalanobis model considered by Nehru as a means to implement the Fabian Society version of socialism.[25][26][27] In addition to Nehru, several pre-independence leaders in colonial India such as Annie Besant—Nehru's mentor and later a president of Indian National Congress – were members of the Fabian Society.[5]
Obafemi Awolowo, who later became the premier of Nigeria's now-defunct Western Region, was also a Fabian member in the late 1940s. It was the Fabian ideology that Awolowo used to run the Western Region during his premiership with great success, although he was prevented from using it in a similar fashion on the national level in Nigeria. It is less known that the founder of Pakistan, Muhammad Ali Jinnah[citation needed], was an avid member of the Fabian Society in the early 1930s. Lee Kuan Yew, the first Prime Minister of Singapore, stated in his memoirs that his initial political philosophy was strongly influenced by the Fabian Society. However, he later altered his views, considering the Fabian ideal of socialism as impractical.[28] In 1993, Lee said:
In the Middle East, the theories of Fabian Society intellectual movement of early-20th-century Britain inspired the Ba'athist vision. The Middle East adaptation of Fabian socialism led the state to control big industry, transport, banks, internal and external trade. The state would direct the course of economic development, with the ultimate aim to provide a guaranteed minimum standard of living for all.[29] Michel Aflaq, widely considered as the founder of the Ba'athist movement, was a Fabian socialist. Aflaq's ideas, with those of Salah al-Din al-Bitar and Zaki al-Arsuzi, came to fruition in the Arab world in the form of dictatorial regimes in Iraq and Syria.[30] Salāmah Mūsā of Egypt, another prominent champion of Arab Socialism, was a keen adherent of Fabian Society, and a member since 1909.[31]
In October 1940, the Fabian Society established the Fabian Colonial Bureau to facilitate research and debate British colonial policy.[32] The Fabian Colonial Bureau strongly influenced the colonial policies of the Attlee government (1945-51).[33] Rita Hinden founded the colonial bureau and was its secretary.[33]
Fabian academics of the late 20th-century included the political scientist Sir Bernard Crick, the economists Thomas Balogh and Nicholas Kaldor and the sociologist Peter Townsend.
In the philosophy of thermal and statistical physics, the Brownian ratchet or Feynman–Smoluchowski ratchet is an apparent perpetual motion machine of the second kind, first analysed in 1912 as a thought experiment by Polish physicist Marian Smoluchowski.[1] It was popularised by American Nobel laureate physicist Richard Feynman in a physics lecture at the California Institute of Technology on May 11, 1962, during his Messenger Lectures series The Character of Physical Law in Cornell University in 1964 and in his text The Feynman Lectures on Physics[2] as an illustration of the laws of thermodynamics. The simple machine, consisting of a tiny paddle wheel and a ratchet, appears to be an example of a Maxwell's demon, able to extract mechanical work from random fluctuations (heat) in a system at thermal equilibrium, in violation of the second law of thermodynamics. Detailed analysis by Feynman and others showed why it cannot actually do this. Sadly people bought the story based on the mechanical idea not on the physical idea where this is the process that generates electricity which is in everything and is the lossless system as the movement causes itself to be caused rather than the idea presented where the cause is implied to be some outside force all physics demonstrations are fantasy as they all neglect to consider what is causing everything and how that force factors into every other thing that happens...Oxygen breaking up with whatever it is bonded with as the material heats up and bonding with Oxygen seeking material when released...the perpetual motion that no hominid alive on the planet is going to even attempt to deny is ever present ever constant ever never ending matrix of matricies...http://www.shodor.org/UNChem/math/matrix/index.html Games!...A simple example goes a long way. We can form water by combing hydrogen gas (H2) and oxygen (O2) in the presence of electricity. The reaction looks like this:
If you do some of the gram molecular weight calculations you will find this:
They're not lost, we just haven't balanced the equation! You might have also noticed that there are two oxygens on the left and only one on the right! We need to get things in the correct proportions for this reaction to be balanced. The balanced reaction looks like this:
This says that we need two hydrogen molecules to combine with one oxygen molecule to form two new water molecules. If we do the math:













.jpg)



Comments
Post a Comment
No Comment