Three points come together to. form a fourth idea
Magnetic field of the Moon

The magnetic field of the Moon is very weak in comparison to that of the Earth; the major difference is the Moon does not have a dipolar magnetic field currently (as would be generated by a geodynamo in its core), so that the magnetization present is varied (see picture) and its origin is almost entirely crustal in location; so it's difficult to compare as a percentage to Earth. But, one experiment discovered that lunar rocks formed 1 - 2.5 billion years ago were created in a field of about 5 microtesla (μT), compared to present day Earth's 50 μT.[1] During the Apollo program several magnetic field strength readings were taken with readings ranging from a low of 6γ (6nT) at the Apollo 15 site to a maximum of 313γ (0.31μT) at the Apollo 16 site,[2] note these readings were recorded in gammas(γ) a now outdated unit of magnetic flux density equivalent to 1nT.
One hypothesis holds that the crustal magnetizations were acquired early in lunar history when a geodynamo was still operating. An analysis of magnetized Moon rocks brought to Earth by Apollo astronauts showed that the Moon must have had a strong (above 110 μT)[3] magnetic field at least 4.25 billion years ago, which then fell to 20 μT level in the 3.6 - 3.1 billion years BP period.[4] The small size of the lunar core, however, is a potential obstacle to promoting that hypothesis to the status of theory. However, single silicate grains with magnetic inclusions from Apollo rocks formed at 3.9, 3.6, 3.3, and 3.2 billion years ago have been shown to be capable of recording strong magnetic fields but do not.[5] This supports the alternative hypothesis that the Moon never had a long-lasting core dynamo, consistent with the lack of energy needed to sustain a field.[6]
It is possible that on an airless body such as the Moon, transient magnetic fields could be generated during large impact events. In particular, study of Apollo impact glass associated with a young, 2 million-year-old crater has yielded a strong magnetization comparable in strength to Earth's magnetic field[5]. This magnetization could not have originated in the lunar core, but is instead consistent with predictions from fields associated with impact plasmas[5].[7] These observations have led to the hypothesis that prior reports of high paleofield strengths from Apollo samples record impacts, not a core dynamo[5,6]. Importantly, the lack of a long-lasting lunar dynamo and paleomagnetosphere should have allowed 3He, water, and other volatile resources acquired from solar winds and Earth’s magnetosphere over some 4 billion years to accumulate in lunar soils [5,6].
It has also been noted that the largest crustal magnetizations appear to be located near the antipodes of the giant impact basins. It has been proposed that such a phenomenon could result from the free expansion of an impact-generated plasma cloud around the Moon in the presence of an ambient magnetic field.[8] For example, the Chandrayaan-1 spacecraft mapped a "mini-magnetosphere" at the Crisium antipode on the Moon's far side, using its Sub-keV Atom Reflecting Analyzer (SARA) instrument. The mini-magnetosphere is 360 km across at the surface and is surrounded by a 300-km-thick region of enhanced plasma flux that results from the solar wind flowing around the mini-magnetosphere.[9]
There is growing evidence that fine particles of moondust might actually float, ejected from the lunar surface by electrostatic repulsion. This could create a temporary nighttime "atmosphere" of dust. The moondust atmosphere might also gather itself into a sort of diaphanous wind. Drawn by differences in global charge accumulation, floating dust would naturally fly from the strongly negative nightside to the weakly negative dayside. This "dust storm" effect would be strongest at the Moon's terminator. Much of these details are still speculative, but the Lunar Prospector spacecraft detected changes in the lunar nightside voltage during magnetotail crossings, jumping from -200 V to -1000 V. Further characterization was done by the Lunar Atmosphere and Dust Environment Explorer orbiter in late 2013.[10][11]
The plasma sheet is a very dynamic structure, in a constant state of motion, so as the Moon orbits through the magnetotail the plasma sheet can sweep across it many times with encounters lasting anywhere from minutes to hours or even days.[12]
Radiation pressure[edit]
The radiation pressure exerted by the large quantity of X-ray photons inside the closed casing might be enough to compress the secondary. Electromagnetic radiation such as X-rays or light carries momentum and exerts a force on any surface it strikes. The pressure of radiation at the intensities seen in everyday life, such as sunlight striking a surface, is usually imperceptible, but at the extreme intensities found in a thermonuclear bomb the pressure is enormous.
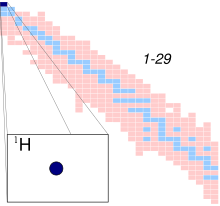
Hydrogen atom
 | |
| General | |
|---|---|
| Symbol | 1H |
| Names | hydrogen atom, 1H, H-1, protium |
| Protons (Z) | 1 |
| Neutrons (N) | 0 |
| Nuclide data | |
| Natural abundance | 99.985% |
| Half-life (t1/2) | stable |
| Isotope mass | 1.007825 Da |
| Spin | 1/2 |
| Excess energy | 7288.969±0.001 keV |
| Binding energy | 0.000±0.0000 keV |
| Isotopes of hydrogen Complete table of nuclides | |
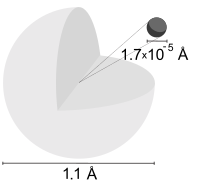
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe.[1]
In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).
Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a theoretical understanding of the states of the hydrogen atom have been important to the history of quantum mechanics, since all other atoms can be roughly understood by knowing in detail about this simplest atomic structure.
Isotopes[edit]
The most abundant isotope, protium (1H), or light hydrogen, contains no neutrons and is simply a proton and an electron. Protium is stable and makes up 99.985% of naturally occurring hydrogen atoms.[2]
Deuterium (2H) contains one neutron and one proton in its nucleus. Deuterium is stable, makes up 0.0156% of naturally occurring hydrogen,[2] and is used in industrial processes like nuclear reactors and Nuclear Magnetic Resonance.
Tritium (3H) contains two neutrons and one proton in its nucleus and is not stable, decaying with a half-life of 12.32 years. Because of its short half-life, tritium does not exist in nature except in trace amounts.
Heavier isotopes of hydrogen are only created artificially in particle accelerators and have half-lives on the order of 10−22 seconds. They are unbound resonances located beyond the neutron drip line; this results in prompt emission of a neutron.
The formulas below are valid for all three isotopes of hydrogen, but slightly different values of the Rydberg constant (correction formula given below) must be used for each hydrogen isotope.
Hydrogen ion[edit]
Lone neutral hydrogen atoms are rare under normal conditions. However, neutral hydrogen is common when it is covalently bound to another atom, and hydrogen atoms can also exist in cationic and anionic forms.
If a neutral hydrogen atom loses its electron, it becomes a cation. The resulting ion, which consists solely of a proton for the usual isotope, is written as "H+" and sometimes called hydron. Free protons are common in the interstellar medium, and solar wind. In the context of aqueous solutions of classical Brønsted–Lowry acids, such as hydrochloric acid, it is actually hydronium, H3O+, that is meant. Instead of a literal ionized single hydrogen atom being formed, the acid transfers the hydrogen to H2O, forming H3O+.
If instead a hydrogen atom gains a second electron, it becomes an anion. The hydrogen anion is written as "H–" and called hydride.
A table with experimental single bonds for carbon to other elements is given below. Bond lengths are given in picometers. By approximation the bond distance between two different atoms is the sum of the individual covalent radii (these are given in the chemical element articles for each element). As a general trend, bond distances decrease across the row in the periodic table and increase down a group. This trend is identical to that of the atomic radius. H bonds with CBond lengths of carbon with other elements[edit]
at a multiple of 6 which puts it squarely in the realm of music defined by the radius of the sun at 432 radiating in all directions at intervals of the twelveth root of two Bond distance of carbon to other elements[1] Bonded element Bond length (pm) Group H 106–112 group 1 Be 193 group 2 Mg 207 group 2 B 156 group 13 Al 224 group 13 In 216 group 13 C 120–154 group 14 Si 186 group 14 Pb 229 group 14 N 147–210 group 15 P 187 group 15 As 198 group 15 Sb 220 group 15 O 143–215 group 16
While Oxygen brings up
the 16th at the 15-45
intrerval/zzzzzzzzz
Pitch (music)

Play top:
Play bottom:
Pitch is a perceptual property that allows sounds to be ordered on a frequency-related scale,[1] or more commonly, pitch is the quality that makes it possible to judge sounds as "higher" and "lower" in the sense associated with musical melodies.[2] Pitch is a major auditory attribute of musical tones, along with duration, loudness, and timbre.[3]
Pitch may be quantified as a frequency, but pitch is not a purely objective physical property; it is a subjective psychoacoustical attribute of sound. Historically, the study of pitch and pitch perception has been a central problem in psychoacoustics, and has been instrumental in forming and testing theories of sound representation, processing, and perception in the auditory system.[4]
Perception[edit]
Pitch and frequency[edit]
Pitch is an auditory sensation in which a listener assigns musical tones to relative positions on a musical scale based primarily on their perception of the frequency of vibration (audio frequency).[5] Pitch is closely related to frequency, but the two are not equivalent. Frequency is an objective, scientific attribute which can be measured. Pitch is the subjective perception of a sound wave by the individual person, which cannot be directly measured. However, this does not necessarily mean that people will not agree on which notes are higher and lower.
The oscillations of sound waves can often be characterized in terms of frequency. Pitches are usually associated with, and thus quantified as, frequencies (in cycles per second, or hertz), by comparing the sounds being assessed against sounds with pure tones (ones with periodic, sinusoidal waveforms). Complex and aperiodic sound waves can often be assigned a pitch by this method.[6][7][8]
According to the American National Standards Institute, pitch is the auditory attribute of sound allowing those sounds to be ordered on a scale from low to high. Since pitch is such a close proxy for frequency, it is almost entirely determined by how quickly the sound wave is making the air vibrate and has almost nothing to do with the intensity, or amplitude, of the wave. That is, "high" pitch means very rapid oscillation, and "low" pitch corresponds to slower oscillation. Despite that, the idiom relating vertical height to sound pitch is shared by most languages.[9] At least in English, it is just one of many deep conceptual metaphors that involve up/down. The exact etymological history of the musical sense of high and low pitch is still unclear. There is evidence that humans do actually perceive that the source of a sound is slightly higher or lower in vertical space when the sound frequency is increased or reduced.[9]
In most cases, the pitch of complex sounds such as speech and musical notes corresponds very nearly to the repetition rate of periodic or nearly-periodic sounds, or to the reciprocal of the time interval between repeating similar events in the sound waveform.[7][8]
The pitch of complex tones can be ambiguous, meaning that two or more different pitches can be perceived, depending upon the observer.[4] When the actual fundamental frequency can be precisely determined through physical measurement, it may differ from the perceived pitch because of overtones, also known as upper partials, harmonic or otherwise. A complex tone composed of two sine waves of 1000 and 1200 Hz may sometimes be heard as up to three pitches: two spectral pitches at 1000 and 1200 Hz, derived from the physical frequencies of the pure tones, and the combination tone at 200 Hz, corresponding to the repetition rate of the waveform. In a situation like this, the percept at 200 Hz is commonly referred to as the missing fundamental, which is often the greatest common divisor of the frequencies present.[10]
Pitch depends to a lesser degree on the sound pressure level (loudness, volume) of the tone, especially at frequencies below 1,000 Hz and above 2,000 Hz. The pitch of lower tones gets lower as sound pressure increases. For instance, a tone of 200 Hz that is very loud seems one semitone lower in pitch than if it is just barely audible. Above 2,000 Hz, the pitch gets higher as the sound gets louder.[11] These results were obtained in the pioneering works by S. Stevens[12] and W. Snow.[13] Later investigations, i.e. by A. Cohen, have shown that in most cases the apparent pitch shifts were not significantly different from pitch‐matching errors. When averaged, the remaining shifts followed the directions of Stevens's curves but were small (2% or less by frequency, i.e. not more than a semitone).[14]
Theories of pitch perception[edit]
Theories of pitch perception try to explain how the physical sound and specific physiology of the auditory system work together to yield the experience of pitch. In general, pitch perception theories can be divided into place coding and temporal coding. Place theory holds that the perception of pitch is determined by the place of maximum excitation on the basilar membrane.
A place code, taking advantage of the tonotopy in the auditory system, must be in effect for the perception of high frequencies, since neurons have an upper limit on how fast they can phase-lock their action potentials.[5] However, a purely place-based theory cannot account for the accuracy of pitch perception in the low and middle frequency ranges. Moreover, there is some evidence that some non-human primates lack auditory cortex responses to pitch despite having clear tonotopic maps in auditory cortex, showing that tonotopic place codes are not sufficient for pitch responses.[15]
Temporal theories offer an alternative that appeals to the temporal structure of action potentials, mostly the phase-lock of action potentials to frequencies in a stimulus. The precise way this temporal structure helps code for pitch at higher levels is still debated, but the processing seems to be based on an autocorrelation of action potentials in the auditory nerve.[16] However, it has long been noted that a neural mechanism that may accomplish a delay—a necessary operation of a true autocorrelation—has not been found.[5] At least one model shows that a temporal delay is unnecessary to produce an autocorrelation model of pitch perception, appealing to phase shifts between cochlear filters;[17] however, earlier work has shown that certain sounds with a prominent peak in their autocorrelation function do not elicit a corresponding pitch percept,[18][19] and that certain sounds without a peak in their autocorrelation function nevertheless elicit a pitch.[20][21] To be a more complete model, autocorrelation must therefore apply to signals that represent the output of the cochlea, as via auditory-nerve interspike-interval histograms.[19] Some theories of pitch perception hold that pitch has inherent octave ambiguities, and therefore is best decomposed into a pitch chroma, a periodic value around the octave, like the note names in Western music—and a pitch height, which may be ambiguous, that indicates the octave the pitch is in.[4]
Just-noticeable difference[edit]
The just-noticeable difference (jnd) (the threshold at which a change is perceived) depends on the tone's frequency content. Below 500 Hz, the jnd is about 3 Hz for sine waves, and 1 Hz for complex tones; above 1000 Hz, the jnd for sine waves is about 0.6% (about 10 cents).[22] The jnd is typically tested by playing two tones in quick succession with the listener asked if there was a difference in their pitches.[11] The jnd becomes smaller if the two tones are played simultaneously as the listener is then able to discern beat frequencies. The total number of perceptible pitch steps in the human hearing range is about 1,400; the total number of notes in the equal-tempered scale, from 16 to 16,000 Hz, is 120.[11]
Aural illusions[edit]
The relative perception of pitch can be fooled, resulting in aural illusions. There are several of these, such as the tritone paradox, but most notably the Shepard scale, where a continuous or discrete sequence of specially formed tones can be made to sound as if the sequence continues ascending or descending forever.
de Broglie wavelength[edit]
In 1923, Louis de Broglie generalized the Planck–Einstein relation by postulating that the Planck constant represents the proportionality between the momentum and the quantum wavelength of not just the photon, but the quantum wavelength of any particle. This was confirmed by experiments soon afterward. This holds throughout the quantum theory, including electrodynamics. The de Broglie wavelength λ of the particle is given by
where p denotes the linear momentum of a particle, such as a photon, or any other elementary particle.
The energy of a photon with angular frequency ω = 2πf is given by
while its linear momentum relates to
where k is an angular wavenumber.
These two relations are the temporal and spatial parts of the special relativistic expression using 4-vectors.
Wavenumber

In the physical sciences, the wavenumber (or wave number), also known as repetency,[1] is the spatial frequency of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber).[2][3][4] It is analogous to temporal frequency, which is defined as the number of wave cycles per unit time (ordinary frequency) or radians per unit time (angular frequency).
In multidimensional systems, the wavenumber is the magnitude of the wave vector. The space of wave vectors is called reciprocal space. Wave numbers and wave vectors play an essential role in optics and the physics of wave scattering, such as X-ray diffraction, neutron diffraction, electron diffraction, and elementary particle physics. For quantum mechanical waves, the wavenumber multiplied by the reduced Planck constant is the canonical momentum.
Wavenumber can be used to specify quantities other than spatial frequency. For example, in optical spectroscopy, it is often used as a unit of temporal frequency assuming a certain speed of light.
Definition[edit]
Wavenumber, as used in spectroscopy and most chemistry fields, is defined as the number of wavelengths per unit distance, typically centimeters (cm−1):
where λ is the wavelength. It is sometimes called the "spectroscopic wavenumber".[1] It equals the spatial frequency.
Momentum
| Momentum | |
|---|---|
 Momentum of a pool cue ball is transferred to the racked balls after collision. | |
Common symbols | p, p |
| SI unit | kg⋅m/s |
Other units | slug⋅ft/s |
| Conserved? | Yes |
| Dimension | |
| Part of a series on |
| Classical mechanics |
|---|
Branches |
Formulations |
Core topics |
Scientists |
In Newtonian mechanics, momentum (pl.: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If m is an object's mass and v is its velocity (also a vector quantity), then the object's momentum p (from Latin pellere "push, drive") is:
In the International System of Units (SI), the unit of measurement of momentum is the kilogram metre per second (kg⋅m/s), which is dimensionally equivalent to the newton-second.
Newton's second law of motion states that the rate of change of a body's momentum is equal to the net force acting on it. Momentum depends on the frame of reference, but in any inertial frame it is a conserved quantity, meaning that if a closed system is not affected by external forces, its total linear momentum does not change. Momentum is also conserved in special relativity (with a modified formula) and, in a modified form, in electrodynamics, quantum mechanics, quantum field theory, and general relativity. It is an expression of one of the fundamental symmetries of space and time: translational symmetry.
Roundabout
Diatomic molecule

Diatomic molecules (from Greek di- 'two') are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (H2) or oxygen (O2), then it is said to be homonuclear. Otherwise, if a diatomic molecule consists of two different atoms, such as carbon monoxide (CO) or nitric oxide (NO), the molecule is said to be heteronuclear. The bond in a homonuclear diatomic molecule is non-polar.

The only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (STP) (or at typical laboratory conditions of 1 bar and 25 °C) are the gases hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), and chlorine (Cl2), and the liquid bromine (Br2).[1]
The noble gases (helium, neon, argon, krypton, xenon, and radon) are also gases at STP, but they are monatomic. The homonuclear diatomic gases and noble gases together are called "elemental gases" or "molecular gases", to distinguish them from other gases that are chemical compounds.[2]
At slightly elevated temperatures, the halogens bromine (Br2) and iodine (I2) also form diatomic gases.[3] All halogens have been observed as diatomic molecules, except for astatine and tennessine, which are uncertain.
Other elements form diatomic molecules when evaporated, but these diatomic species repolymerize when cooled. Heating ("cracking") elemental phosphorus gives diphosphorus (P2). Sulfur vapor is mostly disulfur (S2). Dilithium (Li2) and disodium (Na2)[4] are known in the gas phase. Ditungsten (W2) and dimolybdenum (Mo2) form with sextuple bonds in the gas phase. Dirubidium (Rb2) is diatomic.
Occurrence[edit]
Hundreds of diatomic molecules have been identified[5] in the environment of the Earth, in the laboratory, and in interstellar space. About 99% of the Earth's atmosphere is composed of two species of diatomic molecules: nitrogen (78%) and oxygen (21%). The natural abundance of hydrogen (H2) in the Earth's atmosphere is only of the order of parts per million, but H2 is the most abundant diatomic molecule in the universe. The interstellar medium is dominated by hydrogen atoms.
Molecular geometry[edit]
All diatomic molecules are linear and characterized by a single parameter which is the bond length or distance between the two atoms. Diatomic nitrogen has a triple bond, diatomic oxygen has a double bond, and diatomic hydrogen, fluorine, chlorine, iodine, and bromine all have single bonds.[6]
Magnetic dipole
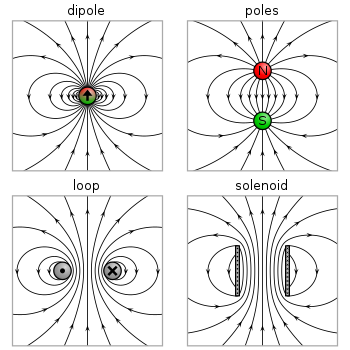
In electromagnetism, a magnetic dipole is the limit of either a closed loop of electric current or a pair of poles as the size of the source is reduced to zero while keeping the magnetic moment constant.
It is a magnetic analogue of the electric dipole, but the analogy is not perfect. In particular, a true magnetic monopole, the magnetic analogue of an electric charge, has never been observed in nature. However, magnetic monopole quasiparticles have been observed as emergent properties of certain condensed matter systems.[2] Moreover, one form of magnetic dipole moment is associated with a fundamental quantum property—the spin of elementary particles.
Because magnetic monopoles do not exist, the magnetic field at a large distance from any static magnetic source looks like the field of a dipole with the same dipole moment. For higher-order sources (e.g. quadrupoles) with no dipole moment, their field decays towards zero with distance faster than a dipole field does.
In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude of torque the object experiences in a given magnetic field. When the same magnetic field is applied, objects with larger magnetic moments experience larger torques. The strength (and direction) of this torque depends not only on the magnitude of the magnetic moment but also on its orientation relative to the direction of the magnetic field. Its direction points from the south pole to north pole of the magnet (i.e., inside the magnet).
The magnetic moment also expresses the magnetic force effect of a magnet. The magnetic field of a magnetic dipole is proportional to its magnetic dipole moment. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse cube of the distance from the object.
Examples of objects or systems that produce magnetic moments include: permanent magnets; astronomical objects such as many planets, including the Earth, and some moons, stars, etc.; various molecules; elementary particles (e.g. electrons); composites of elementary particles (protons and neutrons—as of the nucleus of an atom); and loops of electric current such as exerted by electromagnets.
Earth's magnetic field
| Articles about |
| Electromagnetism |
|---|
 |
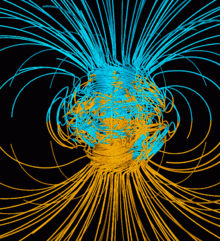
Earth's magnetic field, also known as the geomagnetic field, is the magnetic field that extends from Earth's interior out into space, where it interacts with the solar wind, a stream of charged particles emanating from the Sun. The magnetic field is generated by electric currents due to the motion of convection currents of a mixture of molten iron and nickel in Earth's outer core: these convection currents are caused by heat escaping from the core, a natural process called a geodynamo.
The magnitude of Earth's magnetic field at its surface ranges from 25 to 65 μT (0.25 to 0.65 G).[3] As an approximation, it is represented by a field of a magnetic dipole currently tilted at an angle of about 11° with respect to Earth's rotational axis, as if there were an enormous bar magnet placed at that angle through the center of Earth. The North geomagnetic pole actually represents the South pole of Earth's magnetic field, and conversely the South geomagnetic pole corresponds to the north pole of Earth's magnetic field (because opposite magnetic poles attract and the north end of a magnet, like a compass needle, points toward Earth's South magnetic field, Ellesmere Island, Nunavut, Canada).
While the North and South magnetic poles are usually located near the geographic poles, they slowly and continuously move over geological time scales, but sufficiently slowly for ordinary compasses to remain useful for navigation. However, at irregular intervals averaging several hundred thousand years, Earth's field reverses and the North and South Magnetic Poles respectively, abruptly switch places. These reversals of the geomagnetic poles leave a record in rocks that are of value to paleomagnetists in calculating geomagnetic fields in the past. Such information in turn is helpful in studying the motions of continents and ocean floors. The magnetosphere extends above the ionosphere that is defined by the extent of Earth's magnetic field in space or geospace. It extends several tens of thousands of kilometres into space, protecting Earth from the charged particles of the solar wind and cosmic rays that would otherwise strip away the upper atmosphere, including the ozone layer that protects Earth from harmful ultraviolet radiation.
oxygen
Oxygen (O), nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table. Oxygen is a colourless, odourless, tasteless gas essential to living organisms, being taken up by animals, which convert it to carbon dioxide; plants, in turn, utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere. Oxygen forms compounds by reaction with practically any other element, as well as by reactions that displace elements from their combinations with each other; in many cases, these processes are accompanied by the evolution of heat and light and in such cases are called combustions. Its most important compound is water.

















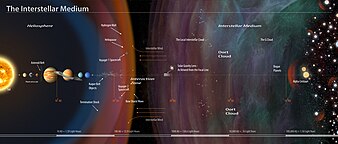


Comments
Post a Comment
No Comment