alkaline water vs alkaline battery
Hydro~lysis (/haɪˈdrɒ~lɪsɪs/)
from Ancient Greek hydro- 'wah~ter' and lye~sis 'to unbind'
is any chemical reaction in which a molecule of water breaks one or more chemical bonds.
The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.[1]
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification.[2]
Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water.[3]
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases.
Nucleophilic describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity.
The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state),
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
but nucleophilicity is a kinetic property, which relates to rates of certain chemical reactions.[1]
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases.
The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding.
Solvations describes the interaction of a solvent with dissolved molecules.
 |
| The Ch~at~h~olics call it Salvation |
A solvation shell or solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute in a solution. When the solvent is water it is called a hydration shell or hydration sphere. The number of solvent molecules surrounding each unit of solute is called the hydration number of the solute.
Ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. When an atom loses an electron to form a cation, the other electrons are more attracted to the nucleus, and the radius of the ion gets smaller. Similarly, when an electron is added to an atom, forming an anion, the added electron increases the size of the electron cloud by interelectronic repulsion.
The ionic radius is not a fixed property of a given ion, but varies with coordination number, spin state and other parameters. Nevertheless, ionic radius values are sufficiently transferable to allow periodic trends to be recognized. As with other types of atomic radius, ionic radii increase on descending a group. Ionic size (for the same ion) also increases with increasing coordination number, and an ion in a high-spin state will be larger than the same ion in a low-spin state. In general, ionic radius decreases with increasing positive charge and increases with increasing negative charge.
An "anomalous" ionic radius in a crystal is often a sign of significant covalent character in the bonding. No bond is completely ionic, and some supposedly "ionic" compounds, especially of the transition metals, are particularly covalent in character. This is illustrated by the unit cell parameters for sodium and silver halides in the table. On the basis of the fluorides, one would say that Ag+ is larger than Na+, but on the basis of the chlorides and bromides the opposite appears to be true.[1] This is because the greater covalent character of the bonds in AgCl and AgBr reduces the bond length and hence the apparent ionic radius of Ag+, an effect which is not present in the halides of the more electropositive sodium, nor in silver fluoride in which the fluoride ion is relatively unpolarizable.
A classic example is when water molecules arrange around a metal ion.
If the metal ion is a cation, the electronegative oxygen atom of the water molecule would be attracted electrostatically to the positive charge on the metal ion. The result is a solvation shell of water molecules that surround the ion. This shell can be several molecules thick, dependent upon the charge of the ion, its distribution and spatial dimensions.
electrons and LIGANDS have the following properties:
The fundamental frequency, often referred to simply as the fundamental (abbreviated as f0 or f1 ), is defined as the lowest frequency of a periodic waveform.[1] In music, the fundamental is the musical pitch of a note that is perceived as the lowest partial present. In terms of a superposition of sinusoids, the fundamental frequency is the lowest frequency sinusoidal in the sum of harmonically related frequencies, or the frequency of the difference between adjacent frequencies.
Wave-like properties:
- Electrons exist as standing waves.
- Electrons do not orbit a nucleus in the manner of a planet orbiting a star.
- Thus :
- the lowest possible energy an electron can take is identical to the fundamental frequency of a wave on a string. Higher energy states are similar to harmonics of that fundamental frequency. A4~432 A5~864 A6~1728
- The electrons are never in a single point location, though the probability of interacting with the electron at a single point can be found from the electron's wave function. The electron's charge acts like it is smeared out in space in a continuous distribution, proportional at any point to the squared magnitude of the electron's wave function.
Part~ICE~icle Imaginary Point~like properties:
- The number of electrons orbiting a nucleus can be only an integer.
- Electrons jump between orbitals like particles. For example, if one photon strikes the electrons, only one electron changes state as a result.
- Electrons retain particle-like properties such as: each wave state has the same electric charge as its electron particle.
- Each wave state has a single discrete spin (spin up or spin down) depending on its superposition.
A number of molecules of solvent are involved in the solvation shell around anions and cations from a dissolved salt in a solvent. Metal ions in aqueous solutions form metal aquo complexes. This number can be determined by various methods like compressibility and NMR measurements among others.
Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density.[1] If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent.
Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration.[2]

Solubility of solid compounds depends on a competition between lattice energy and solvation, including entropy effects related to changes in the solvent structure.[3]
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.
Oxidation states are typically represented by integers which may be positive, zero, or negative. In some cases, the average oxidation state of an element is a fraction, such as 83 for iron in magnetite Fe3O4 (see below). The highest known oxidation state is reported to be +9, displayed by iridium in the tetroxoiridium(IX) cation (IrO+4).[1] It is predicted that even a +10 oxidation state may be achieved by platinum in tetroxoplatinum(X), PtO2+4.[2] The lowest oxidation state is −5, as for boron in Al3BC[3] and gallium in pentamagnesium digallide (Mg5Ga2).
In Stock nomenclature, which is commonly used for inorganic compounds, the oxidation state is represented by a Roman numeral placed after the element name inside parentheses or as a superscript after the element symbol, e.g. Iron(III) oxide.
An endothermic process is a chemical or electro physical process that absorbs heat from its surroundings.[1] In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy H (or internal energy U) of the system.[2] In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings.[1]
Octahedral complexes
[edit]
The Δ splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Three factors affect Δ: the period Integer of Octaves counting Protons of the metal ion of which Hydrogen is the root, the charge of the metal ion counted in integer multiples of hydrogen units +/~1 observed, and the field strength of the complex's ligands as described by the spectrochemical series. Only octahedral complexes of first row transition metals adopt high-spin states.
A spectrochemical series is a list of ligands ordered by ligand "strength", and a list of metal ions based on oxidation number, group and element. For a metal ion, the ligands modify the difference in energy Δ between the d orbitals, called the ligand-field splitting parameter in ligand field theory, or the crystal-field splitting parameter in crystal field theory. The splitting parameter is reflected in the ion's electronic and magnetic properties such as its spin state, and optical properties such as its color and absorption spectrum.
Spectrochemical series of ligands
[edit]The spectrochemical series was first proposed in 1938 based on the results of absorption spectra of cobalt complexes.[1]
Ligand exchange
[edit]A ligand exchange (also called ligand substitution) is a chemical reaction in which a ligand in a compound is replaced by another. Two general mechanisms are recognized: associative substitution or by dissociative substitution.
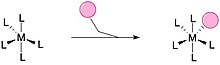
Associative substitution closely resembles the SN2 mechanism in organic chemistry. A typically smaller ligand can attach to an unsaturated complex followed by loss of another ligand. Typically, the rate of the substitution is first order in entering ligand L and the unsaturated complex.[19]

Dissociative substitution is common for octahedral complexes. This pathway closely resembles the SN1 mechanism in organic chemistry. The identity of the entering ligand does not affect the rate.[19]
Ligand–protein binding database
[edit]BioLiP[20] is a comprehensive ligand–protein interaction database, with the 3D structure of the ligand–protein interactions taken from the Protein Data Bank. MANORAA is a webserver for analyzing conserved and differential molecular interaction of the ligand in complex with protein structure homologs from the Protein Data Bank. It provides the linkage to protein targets such as its location in the biochemical pathways, SNPs and protein/RNA baseline expression in target organ.[21]
In order for low spin splitting to occur, the energy cost of placing an electron into an already singly occupied orbital must be less than the cost of placing the additional electron into an eg orbital at an energy cost of Δ. If the energy required to pair two electrons is greater than the energy cost of placing an electron in an eg, Δ, high spin splitting occurs.










Comments
Post a Comment
No Comment